Background: Liver involvement in light chain amyloidosis (AL) is seen in up to 20% of patients and is typically encountered as part of multi-organ involvement. Liver involvement is associated with a poor prognosis. The goal of this study was to assess the prognostic impact of the hepatic response criteria, which were published in 2005 and were not broadly assessed for their prognostic value.
Patients and methods: Ten centers across the USA and Europe participated in this study.AL amyloidosis patients who were diagnosed between 2010 and 2015, had liver involvement with serum alkaline phosphatase (AP) >1.5 upper reference limit (URL), and achieved hematologic response to therapy were included in this study. Hepatic response was assessed at 6, 12, and 24 months from therapy initiation and at best response. Based on a receiver operation characteristic analysis, a 50% reduction in AP was set as the optimal cut point for discrimination of 5-year overall survival (OS) in the 12-months response assessment landmark (area under the curve 0.67). Therefore, hepatic response was defined as a reduction in AP >50% from baseline value in addition to AP normalization, regardless of AP percentage reduction. Hepatic progression was defined as >50% increase in serum AP. OS was assessed from therapy initiation until death or last follow-up and was plotted using the Kaplan-Meier method. The log-rank test used to compare survival between groups. For fixed-time point survival analysis, patients who died before the landmark were excluded. Hepatic response at a fixed time points, when missing, was imputed based on available data from adjacent time points (imputation of hepatic response was performed in 15%, 23%, and 22% of evaluable patients at 6-, 12-, and 24-month landmarks, respectively). Multivariate Cox proportional models were set to determine independent prognostic factors for OS using variables with p-value <0.1 in a univariate analysis. P-values <0.05 were considered significant.
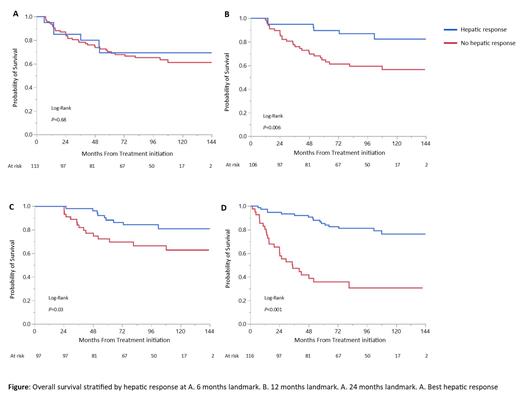
Results: One hundred and sixteen patients (n=116) are included. The median age was 60 years, and the median serum AP was X2.6 URL. Concomitant kidney involvement was present in 63.8% of patients, while coexisting heart involvement was seen in 56% of patients. Hematologic VGPR or better was achieved in 69% of patients. AP decrease deepened with time, with a median reduction at 6-, 12-, 24-months and best response being 22%, 34%, 53%, and 56%, respectively. Overall, 65% of patients (n=75) achieved hepatic response, of whom 71 patients based on reduction in AP >50% and 4 patients for reaching AP within normal reference but not meeting >50% reduction in AP (40- 46% reduction in AP). The median time to hepatic response was 13.8 months, longer for patients undergoing autologous stem cell transplantation (ASCT) as their primary therapy compared to non-transplant therapies (24 vs 12.6 months, P=0.1). With a median follow-up of 105 months, 42 patients died (36.2% of the study cohort). The 2-, 5- and 10-year OS rates were 83%, 67%, and 61%, respectively. Achievement of hepatic response as early as 12 months and at best response was associated with a longer survival (Figure). Hepatic response was an independent prognostic factor in a multivariate analysis, after adjusting for other known prognostic factors (age, cardiac stage, hematologic response, and primary therapy). Predictors of hepatic response include higher baseline AP level, lower total bilirubin, hematologic ≥VGPR, and cardiac and renal responses, when applicable. Hepatic progression was borderline associated with inferior OS (5-year OS 47% vs 70% among non-progressors, P=0.08).
Conclusions: Hepatic response and progression measured by the change in alkaline phosphatase are prognostic in AL amyloidosis. These criteria are independent of other prognostic markers, and thus may have clinical implications for patients' care.
Disclosures
Muchtar:Protego: Consultancy. Palladini:Argobio, GSK: Consultancy; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Prothena: Consultancy, Honoraria; Sebia: Honoraria; Siemens: Honoraria. Dispenzieri:Oncopeptides, Sorento: Consultancy; Alnylam, Bristol-Myers Squibb, Janssen, Pfizer, Takeda: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Hegenbart:Alnylam: Honoraria, Speakers Bureau; Prothena: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Kastritis:Janssen: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Honoraria. Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Szalat:Jansse Pharmaceuticals: Other: Participation to one advisory board. Liedtke:Caelum: Other: Grants or contracts; Janssen: Other: Grants or contracts; Participation on a Data Safety Monitoring Board or Advisory Board; Seagen: Other: Grants or contracts; Adaptive: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Kite: Other: Participation on a Data Safety Monitoring Board or Advisory Board; BMS: Other: Grants or contracts; Participation on a Data Safety Monitoring Board or Advisory Board; Allogene: Other: Grants or contracts; Abbvie: Other: Grants or contracts. Landau:Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding; Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria. Lentzsch:Caelum Biosciences: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: January 1, 2041; Clinical Care Options: Honoraria; Celgene: Research Funding; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Regeneron: Honoraria; Sanofi: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bladé:Sanofi: Other: Honoraria for lectures; Amgen: Other: Honoraria for lectures; Celgene/Bristol Myers Squibb: Other: Honoraria for lectures; Janssen: Other: Honoraria for lectures. Wechalekar:Takeda: Other: Travel support; Alexion: Honoraria; Janssen: Honoraria; GSK: Honoraria; Attralus: Honoraria; Alexion, Attralus, Janssen, Prothena.: Consultancy. Gertz:AbbVie: Honoraria; Aptitude: Honoraria; Ashfield: Honoraria, Research Funding; Celgene: Honoraria; Ionis/Akcea: Honoraria; Janssen: Honoraria; Johnson & Johnson: Honoraria; Prothena: Honoraria; Sanofi: Honoraria; Sorrento: Honoraria; Juno: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal